Research Areas
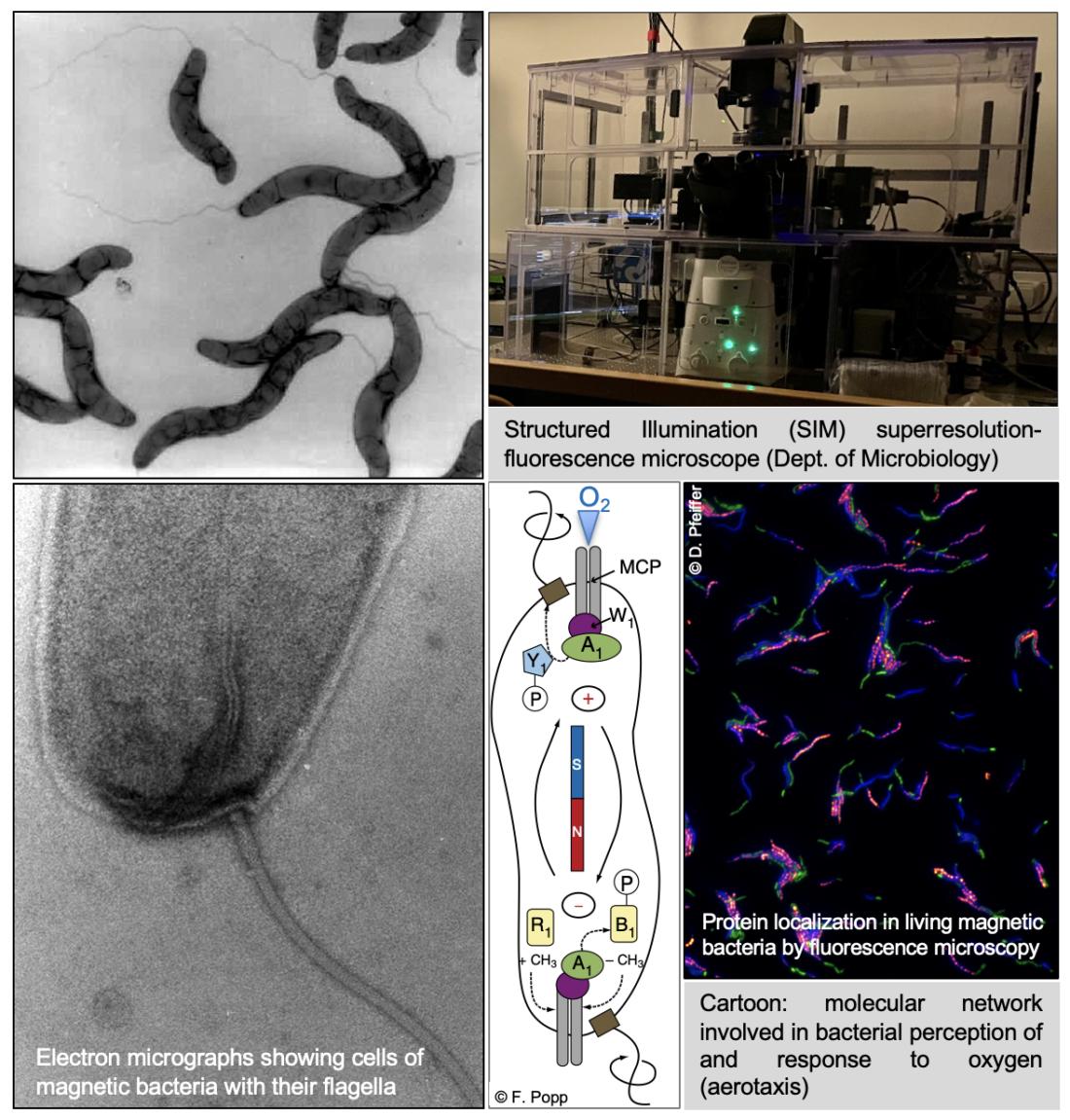
Molecular mechanisms of bacterial magneto-aerotaxis
In magnetotactic bacteria, magnetosomes serve as cellular "sensors" for the passive alignment of cells to the Earth's magnetic field. The combination of active swimming along the geomagnetic field lines (by means of rotating flagella) with a very sensitive aerotaxis enables the bacteria to find regions of optimal oxygen content in aquatic sediments. We explore the underlying molecular mechanisms and cell biology of this intriguing magneto-aerotaxis phenomenon.
Additional information:
Pfeiffer, D., M. Toro-Nahuelpan, R.P. Awal, F.D. Müller, M. Bramkamp, J.M. Plitzko, and D. Schüler. 2020. A bacterial cytolinker couples positioning of magnetic organelles to cell shape control. Proc. Natl. Acad. Sci. USA, doi: 10.1073/pnas.2014659117.
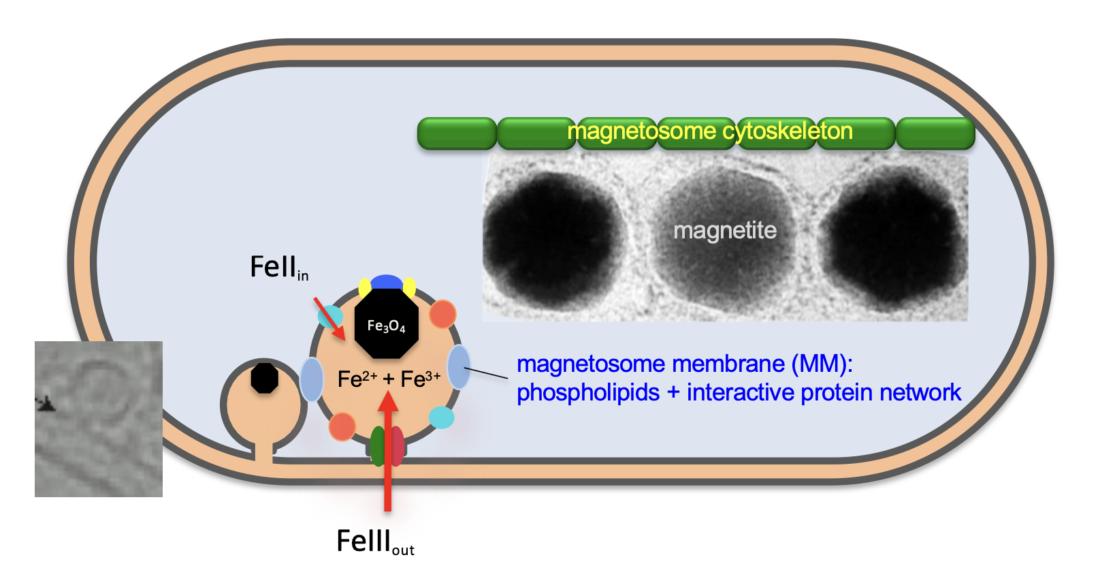
Molecular genetic analysis of magnetosome biosynthesis
The formation of magnetosomes is orchestrated by more than 30 genes. Their products control the biosynthesis of intracellular membrane vesicles, the targeting of specific proteins, the uptake of iron, and the biomineralization of magnetite crystals. Our research aims at unravelling the molecular mechanisms of this complex biosynthesis process.
Additional information:
Uebe, R. and D. Schüler. 2016. Magnetosome biogenesis in magnetotactic bacteria. Nature Rev Microbiol, 14:621–637, doi: 10.1038/nrmicro.2016.99.
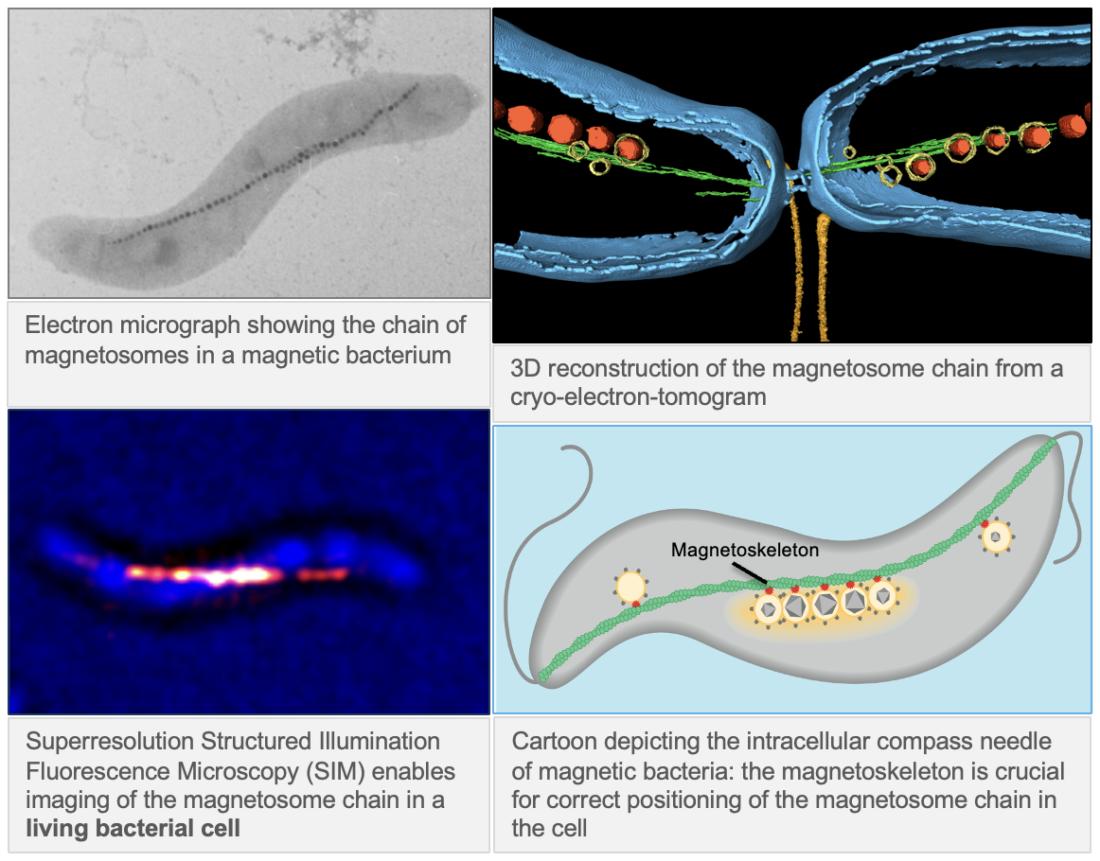
Cell biology of magnetotactic bacteria
In order to build an optimal magnetic field sensor, single magnetosome crystals are lined up in a chain. This arrangement endows the cell with an instrument similar to a compass needle and allows passive alignment of the cell to the the weak geomagnetic field. Formation and positioning of the chain within the bacterial cell occurs along a surprisingly complex network, the so-called "magnetoskeleton", which is established by different cytoskeletal proteins, among them bacterial actins.
Additional information:
Müller, F., D. Schüler, and D. Pfeiffer. 2020. A compass to boost navigation - cell biology of bacterial magnetotaxis. Journal of Bacteriology, doi: 10.1128/JB.00398-20.
Toro-Nahuelpan, M., G. Giacomelli, O. Raschdorf, S. Borg, J. M. Plitzko, M. Bramkamp, D. Schüler, and F. D. Müller. 2019. MamY is a membrane-bound protein that aligns magnetosomes and the motility axis of helical magnetotactic bacteria. Nature Microbiol 4, doi: 10.1038/s41564-019-0512-8.
Background story - Press release - Cover Nature Microbiology
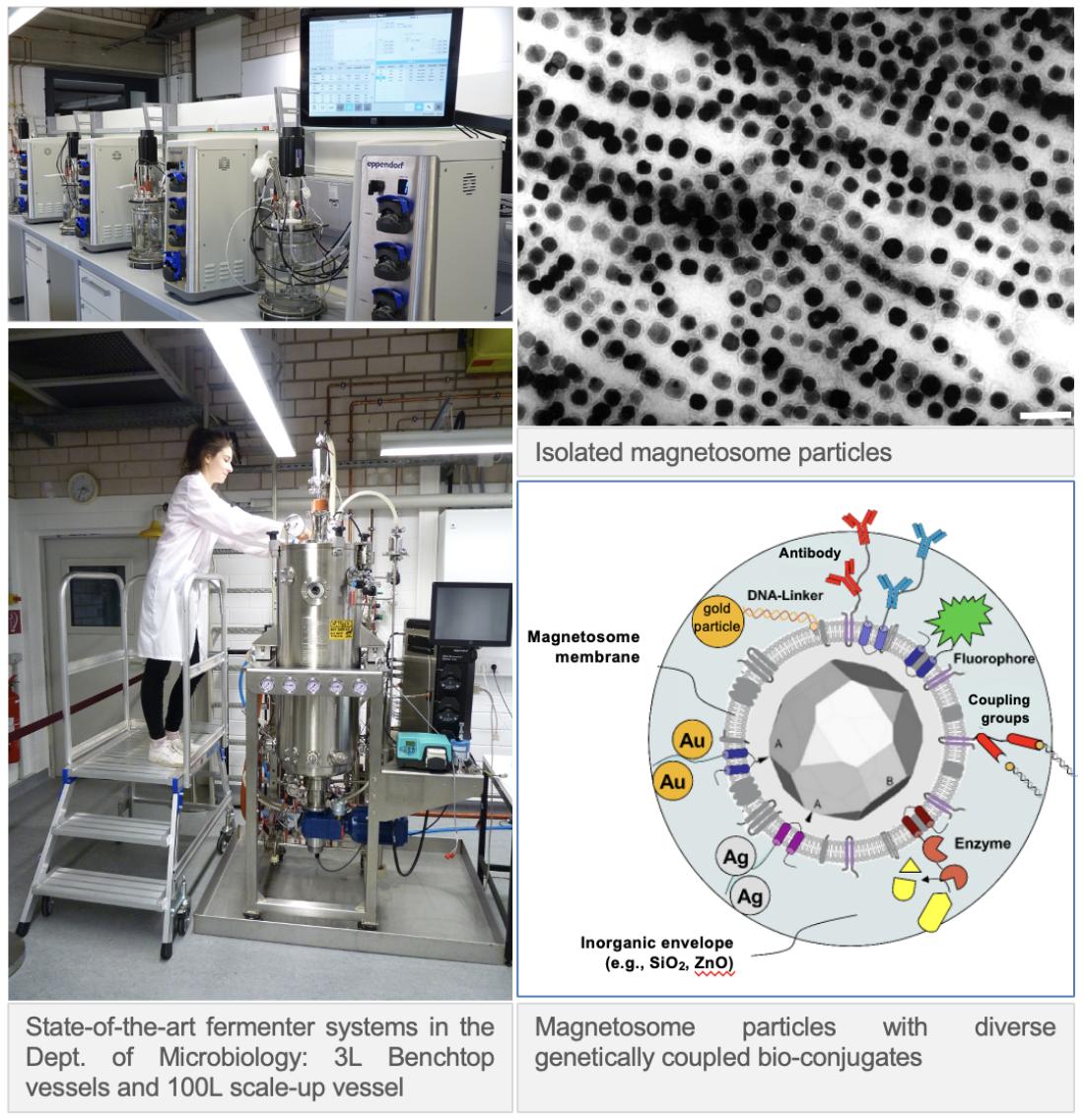
Biotechnology: Generation, engineering and functionalization of magnetic nanoparticles
Apart from their biological function as cellular magnetic field sensors, magnetosomes constitute also magnetic nanoparticles with extraordinary material properties. We broadly fathom options to furnish magnetosomes with additional useful functions for potential applications in research and biomedicine by genetic coupling with diverse biomolecules.
To harness magnetotactic bacteria as production hosts for magnetic nanoparticles, we develop and establish novel methods for mass cultivation and high-yield production and purification of magnetosomes in collaboration with the Dept. for Process Biotechnology.
Moreover, magnetotactic bacteria have recently been revealed as producers of interesting pharmacological biocompounds with cytotoxic activity.
Additional information:
Mickoleit, F., C. Lanzloth and D. Schüler. 2020. A Versatile Toolkit for Controllable and Highly Selective Multifunctionalization of Bacterial Magnetic Nanoparticles. Small, doi: 10.1002/smll.201906922. Pressemitteilungen
Riese, C.N., R. Uebe, S. Rosenfeldt, A.S. Schenk, V. Jérôme, R. Freitag and D. Schüler. 2020. An automated oxystat fermentation regime for microoxic cultivation of Magnetospirillum gryphiswaldense. Microbial Cell Factories, doi: 10.1186/s12934-020-01469-z.
Awal, R.P.*, P.A. Haack*, C.D. Bader, C.N. Riese, D. Schüler#, and R. Müller#. 2021. Sesbanimide R, a novel cytotoxic polyketide produced by magnetotactic bacteria. mBio, https://doi.org/10.1128/mBio.00591-21. *These authors contributed equally to this work. #corresponding authors.
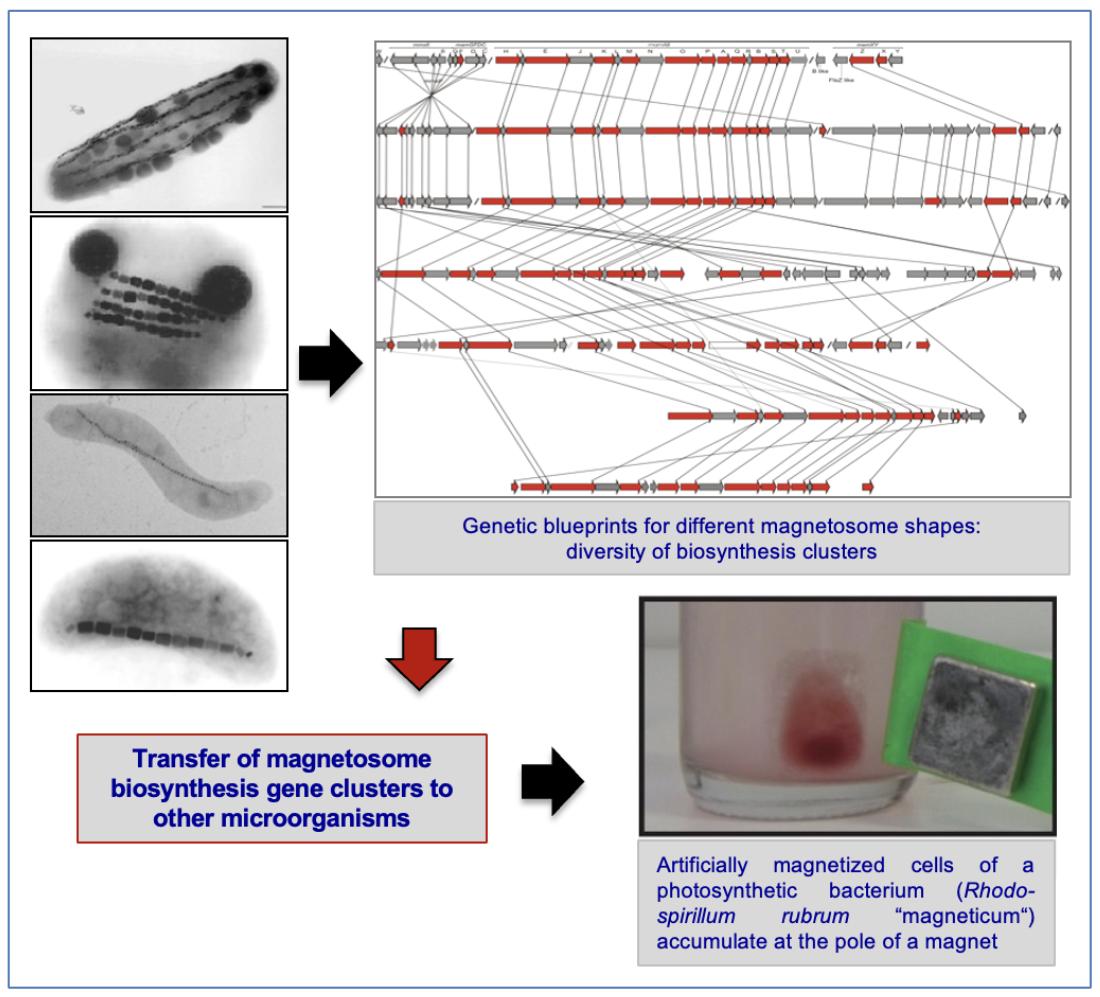
Synthetic biology of magnetosome biosynthesis
Methods of modern genome analysis and molecular genetics provide the exciting possibility to magnetize foreign, naturally non-magnetic microorganisms by transplanting mix-and-match building blocks of diverse magnetosome biosynthesis blueprints, thereby generating "designer" magnetosomes with novel and tailored material properties.
Additional information:
Zwiener, T., M.V. Dziuba, F. Mickoleit, C. Rückert, T. Busche, J. Kalinowski, R. Uebe, and D. Schüler. 2021. Towards a 'chassis' for bacterial magnetosome biosynthesis: Genome streamlining of Magnetospirillum gryphiswaldense by multiple deletions. Microbial Cell Factories, doi: 10.1186/s12934-021-01517-2.
Dziuba, M. V., T. Zwiener, R. Uebe and D. Schüler. 2020. Single-step transfer of biosynthetic operons endows a non-magnetotactic Magnetospirillum strain from wetland with magnetosome biosynthesis. Environmental Microbiology, doi: 10.1111/1462-2920.14950.
Kolinko, I., A. Lohße, S. Borg, O. Raschdorf, C. Jogler, Q. Tu, M. Pósfai, É. Tompa, J. M. Plitzko, A. Brachmann, G. Wanner, R. Müller, Y. Zhang, and D. Schüler. 2014. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nature Nanotech. 9:193–197.