ERC Advanced Grant Syntomagx

SYNTOMAGX
A synthetic biology approach for magnetization of foreign organisms by genetic engineering and transplantation of bacterial magnetosome biosynthesis
Project duration: 2016 - 2022
Project Description
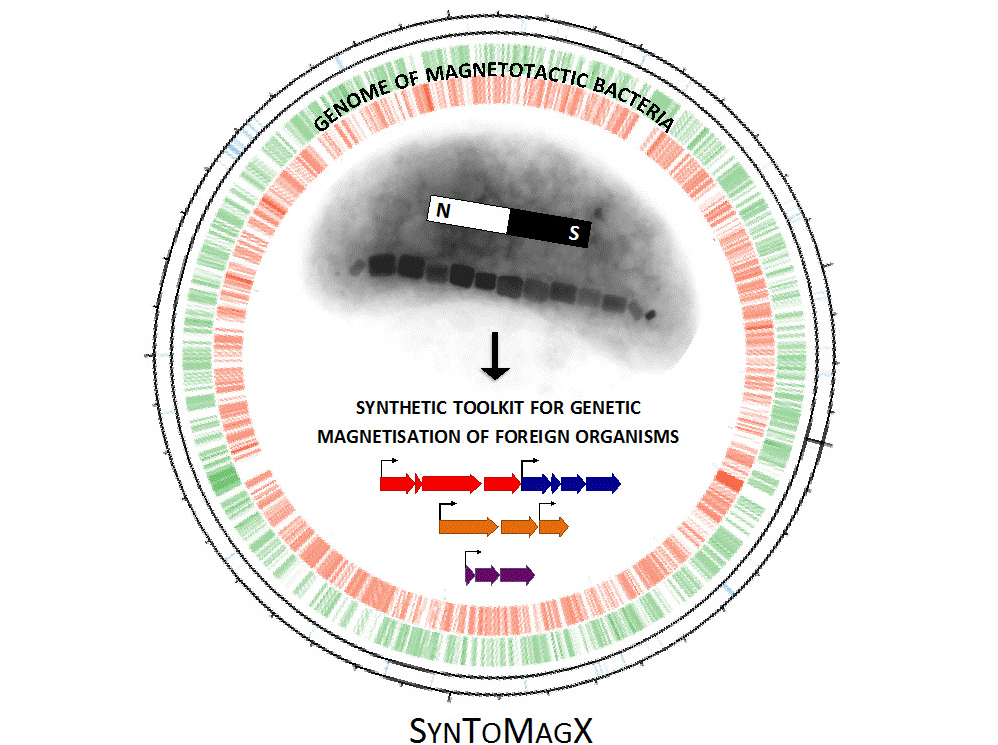
An immensely valuable asset to the field of synthetic biology would be a means to genetically endow magnetism to living organisms, which is still an unsolved challenge due to the lack of appropriate tools. In contrast, biomagnetism is innate to magnetotactic bacteria, mud-dwelling microbes which as geomagnetic sensors biomineralize iron nanocrystals with texceptional properties, the magnetosomes.
However, transplantation of magnetosome biosynthesis has remained unachieved for many years, owing to its complexity and lack of knowledge of genetic determinants. Recently, our lab discovered relevant biosynthetic gene clusters and for the first time succeeded in expressing them in a foreign bacterium. Inspired by this major breakthrough, we now will follow a step change approach for endogenous magnetization of diverse organisms based on bacterial magnetosome biosynthesis. By combining systematic genetic reduction with bottom-up redesign we will first minimize the pathway to make it universally portable. We will then aim to reprogram other bacteria into a chassis for plug-in expression of diverse magnetosome gene sets.
By harnessing determinants of structurally diverse magnetosomes from various bacteria, we will reconfigure the pathway for mix-and-match generation of designer nanoparticles with tuned magnetic properties. Finally, we will attempt to reconstitute key parts of magnetosome formation in eukaryotic hosts by using yeast mitochondria as a universal model. The overall aim is to generate a versatile synthetic toolkit for genetic magnetization of different organisms. This would represent a quantum leap with tremendous impact on various fields of biomedical research and biotechnology. It might be exploited for bioproduction of tailored magnetic nanomaterials with novel and tunable properties. It could be further utilized to generate intracellular labels, tracers and actuators for magnetic manipulation and analysis of cells and organisms in the emerging field of magnetogenetics.
Principal Investigator: Prof. Dr. Dirk Schüler
Publications
Marina V. Dziuba, Anja Paulus, Lukas Schramm, Ram Prasad Awal, Mihály Pósfai, Caroline L. Monteil, Stéphanie Fouteau, René Uebe, and Dirk Schüler. 2022. Silent Gene Clusters Encoding Magnetic Organelle Biosynthesis in a Non-Magnetotactic Phototrophic Bacterium. ISME Journal, https://doi.org/10.1038/s41396-022-01348-y.
Cornelius N. Riese, Manuel Wittchen, Valérie Jérome, Ruth Freitag, Tobias Busche, Jörn Kalinowski, and Dirk Schüler. 2022. The transcriptomic landscape of Magnetospirillum gryphiswaldense during magnetosome biomineralization. BMC Genomics, 23, article no. 699, https://doi.org/10.1186/s12864-022-08913-x.
Mittmann, E#., Mickoleit, F.#, D. Maier, S. Stäbler, M. Klein, Chr. Niemeyer, K. Rabe*, and D. Schüler*. 2022. A Magnetosome-Based Platform for Flow Biocatalysis. ACS Applied Materials & Interfaces, doi: https://doi.org/10.1021/acsami.2c03337. #These authors contributed equally to this work. *corresponding authors.
Toro-Nahuelpan, M., J.M. Plitzko, D. Schüler, and D. Pfeiffer. 2021. In vivo architecture of the polar organizing protein Z (PopZ) meshwork in the Alphaproteobacteria Magnetospirillum gryphiswaldense and Caulobacter crescentus. Journal of Molecular Biology, doi: 10.1016/j.jmb.2021.167423. Perspective article: https://doi.org/10.1016/j.jmb.2022.167521 - cover image: https://www.journals.elsevier.com/journal-of-molecular-biology/covers-gallery/cover-of-volume-434-issue-7
Dziuba, M., C. Riese, L. Borgert, M. Wittchen, T. Busche, J. Kalinowski, R. Uebe, and D. Schüler. 2021. The Complex Transcriptional Landscape of Magnetosome Gene Clusters in Magnetospirillum gryphiswaldense. mSystems, 6:e00893-21. doi: org/10.1128/mSystems.00893-21.
Mickoleit, F., S. Rosenfeldt, M. Toro-Nahuelpan, M. Schaffer, A.S. Schenk, J.M. Plitzko, and D. Schüler. 2021. High-Yield Production, Characterization and Functionalization of Recombinant Magnetosomes in the Synthetic Bacterium Rhodospirillum rubrum "magneticum". Advanced Biology, doi: 10.1002/adbi.202101017.
Mickoleit, F., C. Jörke, S. Geimer, D.S. Maier, J.P. Müller, J. Demut, Chr. Gräfe, D. Schüler*, and J.H. Clement*. 2021. Biocompatibility, uptake and subcellular localization of bacterial magnetosomes in mammalian cells. Nanoscale Advances, doi: 10.1039/D0NA01086C.
De Vincentiis, S., A. Falconieri, F. Mickoleit, V. Cappello, D. Schüler, and V. Raffa. 2021. Induction of axonal outgrowth in mouse hippocampal neurons via bacterial magnetosomes. International Journal of Molecular Sciences, doi: 10.3390/ijms22084126.
Awal, R.P.*, P.A. Haack*, C.D. Bader, C.N. Riese, D. Schüler, and R. Müller. 2021. Sesbanimide R, a novel cytotoxic polyketide produced by magnetotactic bacteria. mBio, doi.org/10.1128/mBio.00591-21; *These authors contributed equally to this work.
Zwiener, T., M.V. Dziuba, F. Mickoleit, Chr. Rückert, T. Busche, J. Kalinowski, R. Uebe, and D. Schüler. 2021. Towards a 'chassis' for bacterial magnetosome biosynthesis: Genome streamlining of Magnetospirillum gryphiswaldense by multiple deletions. Microbial Cell Factories, doi.org/10.1186/s12934-021-01517-2.
Zwiener, T., F. Mickoleit, M.V. Dziuba, Chr. Rückert, T. Busche, J. Kalinowski, D. Faivre, R. Uebe, and D. Schüler. 2021. Identification and elimination of genomic regions irrelevant for magnetosome biosynthesis by large-scale deletion in Magnetospirillum gryphiswaldense. BMC Microbiology, doi: org/10.1186/s12866-021-02124-2.
Pfeiffer, D., M. Toro-Nahuelpan, R.P. Awal, F.D. Müller, M. Bramkamp, J.M. Plitzko, and D. Schüler. 2020. A bacterial cytolinker couples positioning of magnetic organelles to cell shape control. Proc. Natl. Acad. Sci. USA, doi.org/10.1073/pnas.2014659117.
Riese, C.N., R. Uebe, S. Rosenfeldt, A.S. Schenk, V. Jérôme, R. Freitag and D. Schüler. 2020. An automated oxystat fermentation regime for microoxic cultivation of Magnetospirillum gryphiswaldense. Microbial Cell Factories, doi.org/10.1186/s12934-020-01469-z.
Silva, K.T.*, M. Schüler*, F. Mickoleit, T. Zwiener, F.D. Müller, R.P. Awal, A. Weig, A. Brachmann, R. Uebe, and D. Schüler. 2020. Genome-wide identification of essential and auxiliary gene sets for magnetosome biosynthesis in Magnetospirillum gryphiswaldense. mSystems, doi: 10.1128/mSystems.00565-20. *These authors contributed equally to this work.
Pfeiffer, D., J. Herz, J. Schmiedel, F. Popp, and D. Schüler. 2020. Spatiotemporal organization of chemotaxis pathways in Magnetospirillum gryphiswaldense. Applied and Environmental Microbiology, doi: 10.1128/AEM.02229-20.
Müller, F., D. Schüler, and D. Pfeiffer. 2020. A compass to boost navigation - cell biology of bacterial magnetotaxis, Journal of Bacteriology, doi: 10.1128/JB.00398-20.
Rosenfeldt, S.#, F. Mickoleit#, C. Jörke, J.H. Clement, S. Markert, V. Jérôme, St. Schwarzinger, R. Freitag, D. Schüler, R. Uebe*, and A. S. Schenk*. 2020. Towards standardized purification of bacterial magnetic nanoparticles for future in vivo applications, Acta Biomaterialia, https://doi.org/10.1016/j.actbio.2020.07.042 *corresponding authors, #these authors contributed equally to this work.
Schüler, D., C. L. Monteil and Chr. Lefevre. 2020. Microbe of the Month: Magnetospirillum gryphiswaldense. Trends in Microbiology, https://doi.org/10.1016/j.tim.2020.06.001.
Dziuba, M.V., T. Zwiener, R. Uebe and D. Schüler. 2020. Single-step transfer of biosynthetic operons endows a non-magnetotactic Magnetospirillum strain from wetland with magnetosome biosynthesis. Environmental Microbiology, https://doi.org/10.1111/1462-2920.14950.
Mickoleit, F., C. Lanzloth and D. Schüler. 2020. A versatile toolkit for controllable and highly selective multifunctionalization of bacterial magnetic nanoparticles. Small, doi: 10.1002/smll.201906922. Press releases.
Mickoleit, F., V. Jérôme, R. Freitag and D. Schüler. 2020. Bacterial Magnetosomes as novel platform for the presentation of immunostimulatory, membrane-bound ligands in cellular biotechnology. Advanced Biosystems, https://doi.org/10.1002/adbi.201900231.
Pfeiffer, D. and D. Schüler. 2020. Quantifying the benefit of a dedicated 'magnetoskeleton' in bacterial magnetotaxis by live-cell motility tracking and soft agar swimming assay. Applied and Environmental Microbiology 86: e01976-19. https://doi.org/10.1128/AEM.01976-19.
Rosenfeldt, S., C. Riese, F. Mickoleit, D. Schüler, and A. Schenk. 2019. Probing the nanostructure and arrangement of bacterial magnetosomes by small-angle X-ray scattering. Applied and Environmental Microbiology, doi: 10.1128/AEM.01513-19.
Toro-Nahuelpan, M., G. Giacomelli, O. Raschdorf, S. Borg, J. M. Plitzko, M. Bramkamp, D. Schüler, and F. D. Müller. 2019. MamY is a membrane-bound protein that aligns magnetosomes and the motility axis of helical magnetotactic bacteria. Nature Microbiology, doi: 10.1038/s41564-019-0512-8. Background story - Press release - Cover Nature Microbiology, Volume 4 Issue 11, November 2019.
Toro-Nahuelpan, M., L. Corrales-Guerrero, T. Zwiener, M. Osorio-Valeriano, F. D. Müller, J. M. Plitzko, M. Bramkamp, M. Thanbichler, and D. Schüler. 2019. A gradient-forming MipZ protein mediating the control of cell division in the magnetotactic bacterium Magnetospirillum gryphiswaldense. Molecular Microbiology, doi: 10.1111/mmi.14369.
Uebe, R., F. Ahrens, J. Stang, K. Jaeger, L. Boettger, Chr. Schmidt, B. Matzanke, and D. Schüler. 2019. Bacterioferritin of Magnetospirillum gryphiswaldense is a heterotetraeicosameric complex composed of functionally distinct subunits but is not involved in magnetite biomineralization, mBio, doi: 10.1128/mBio.02795-18.
Pfeiffer, D., M. Toro-Nahuelpan, M. Bramkamp, J. Plitzko, and D. Schüler. 2019. The polar organizing protein PopZ is fundamental for proper cell division and segregation of cellular content in Magnetospirillum gryphiswaldense. mBio, doi.org/10.1128/mBio.02716-18.
Mickoleit, F., Chr.B. Borkner, M. Toro-Nahuelpan, H.M. Herold, D.S. Maier, J. Plitzko, T. Scheibel, and D. Schüler. 2018. In vivo coating of bacterial magnetic nanoparticles by magnetosome expression of spider silk-inspired peptides. Biomacromolecules, doi.org/10.1021/acs.biomac.7b01749. Fulltext available at https://epub.uni-bayreuth.de/4312/
Mickoleit, F. and D. Schüler. 2018. Generation of nanomagnetic biocomposites by genetic engineering of bacterial magnetosomes. Bioinspired, Biomimetic and Nanobiomaterials, doi.org/10.1680/jbibn.18.00005.
Uebe, R., N. Keren-Khadmy, N. Zeytuni, E. Katzmann, Y. Navon, G. Davidov, R. Bitton, J. Plitzko, D. Schüler, and R. Zarivach. 2018. The dual role of MamB in magnetosome membrane assembly and magnetite biomineralization. Molecular Microbiology, doi.org/10.1111/mmi.13899. Fulltext available at https://epub.uni-bayreuth.de/4319/
Mickoleit, F. and D. Schüler. 2017. Generation of multifunctional magnetic nanoparticles with amplified catalytic activities by genetic expression of enzyme arrays on bacterial magnetosomes. Advanced Biosystems, doi.org/10.1002/adbi.201700109.
Raschdorf, O., F. Bonn, N. Zeytuni, R. Zarivach, D. Becher, and D. Schüler. 2017. A quantitative assessment of the membrane-integral sub-proteome of a bacterial magnetic organelle. Journal of Proteomics, doi.org/10.1016/j.jprot.2017.10.007. Fulltext available at https://epub.uni-bayreuth.de/4320/
Uebe, R. and D. Schüler. 2016. Magnetosome biogenesis in magnetotactic bacteria. Nature Reviews Microbiology, doi.org/10.1038/nrmicro.2016.99.
